Recommendations on Acute Kidney Injury Biomarkers

Acute kidney injury (AKI) is common in hospitalized adults and children and associated with serious complications and high health care costs. Traditionally, 2 functional biomarkers, serum creatinine (sCr) and urine output, are used to define AKI, but these markers are limited by delayed changes following kidney injury and have low sensitivity and specificity. Several novel biomarkers have been shown to detect AKI earlier and are more sensitive than sCr. For any prevention strategies to be effective, patients with high risk need to be identified before kidney insults result in kidney damage, and AKI needs to be diagnosed as early as possible.
In 2011, the 10th Acute Disease Quality Initiative (ADQI) meeting focused on AKI biomarkers and their application in clinical practice. The expert committee concluded that the evidence for AKI biomarkers was limited and insufficient for recommendations and that more research and strategies toward the adoption of biomarkers in clinical practice were needed. Subsequently, new AKI biomarkers have been discovered, clinical trials have been completed, and some biomarkers have gained official regulatory approval. In 2019, an ADQI meeting was called to review this new evidence and to develop recommendations regarding AKI risk assessment, prediction, prevention, diagnosis, management, and kidney recovery for practicing clinicians and researchers.
At the 23rd Acute Disease Quality Initiative meeting, a meeting of 23 international experts in critical care, nephrology, and related specialties, the panel focused on 4 broad areas, as follows:
- AKI risk assessment,
- AKI prediction and prevention,
- AKI diagnosis, etiology, and management and
- AKI progression and kidney recovery.
The panel developed 11 consensus statements for biomarker use and 14 research recommendations.
The key suggestions were that a combination of damage and functional biomarkers, along with clinical information, be used to identify high-risk patient groups, improve the diagnostic accuracy of AKI, improve processes of care, and assist the management of AKI.
Current evidence from clinical studies supports the use of new biomarkers in prevention and management of AKI.
NGAL (Neutrophil gelatinaseassociated lipocalin), analysed either in urine orplasma, is listed among the AKI biomarkers, which can contribute in diagnosis of AKI and assessing severity of AKI.
The discovery of specific biomarkers of kidney injury has enabled a more precise delineation of the pathophysiology, site, mechanisms, and severity of injury. Some patients with positive damage biomarkers do not fulfill traditional AKI criteria yet have worse outcomes.
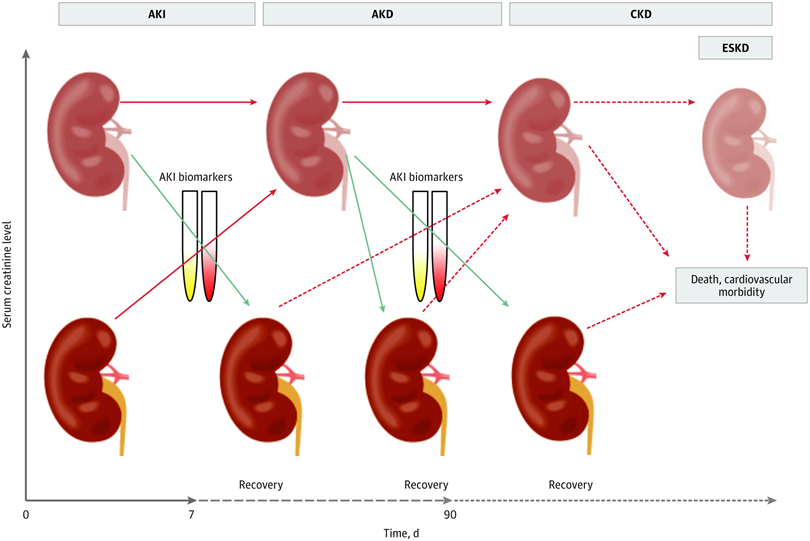
The group proposed that clinical information enriched by damage and functional biomarkers could lead to more sensitive AKI definitions.Therefore, a modification of KDIGO stage 1 AKI has been suggested to reflect 3 substages (ie, 1S, 1A, and 1B) and to subcategorize stages 2 and 3 AKI by presence of biomarkers.

Stage 1S identifies an early stage when there is evidence of kidney injury that is not detected by creatinine and urine output criteria. A concept of subclinical AKI has therefore been inroduced in the proposed AKI definition and staging.